Unique Animal Models

Figure: (A) Mdx mouse diaphragm with early inflammatory phase (F4/80 stained macrophages are shown), followed by (B) severe fibrosis phase (Sirius red of collagen). (C) Acute cardiotoxin-induced diaphragm injury model showing Procion dye uptake in fibers with membrane injury, followed by (D) intense diaphragm muscle regeneration (embryonic myosin heavy chain staining). (E) Mdx mice are injected with retrovirally-engineered mesenchymal stromal cells (MSCs) to create a secretory “organoid” which chronically releases a CCR2-inhibiting molecule into the bloodstream.
Primary Cell Cultures

Primary myogenic cell cultures are derived from satellite cells obtained from isolated single myofibers, thereby ensuring maximal purity. Bone marrow-derived macrophage cultures are also generated from our different experimental models in order to better understand the cross-talk between myogenic and hematopoietic cells.
Figure: (A) Immunofluorescence of HMGB1 (green) and LAMP1 (red) in differentiated myotube cultures. (B) Bone marrow-derived macrophages treated with Interferon-gamma or (C) Interleukin-4.
In Vivo Gene Transfer
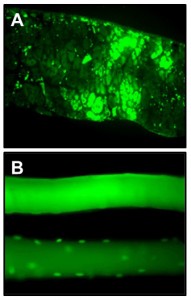
Different vector systems (viral and non-viral) are used to perform in vivo gene transfer to the diaphragm and other muscles.
Figure: (A) Green fluorescent protein (GFP) expression in the diaphragm at 5 days after in vivo gene transfection via plasmid electroporation. (B) Single fiber expression of GFP after in vivo transfection of a standard GFP expression plasmid (top panel) or a plasmid with GFP linked to a nuclear localization sequence (bottom panel).
Laser Capture Microdissection

Laser Capture Microdissection (LCM) allows for localized laser-guided isolation of cellular material from tissue sections while maintaining its biological integrity.
Figure: (A) The centrally nucleated muscle fiber was (B) removed from the tissue section by LCM, and subsequently analyzed using quantitative PCR (see below).
Droplet Digital™ PCR (DDPCR™)

Droplet Digital™ PCR allows for greater precision and reliability in quantifying gene expression on very small samples. This property of DDPCR™ makes the technique a perfect complement to the Laser Capture Microdissection method.
Figure: Schematic representation of the DDPCR™ technology.
Flow Cytometry

Flow cytometry is employed to characterize the phenotype of cells within skeletal muscle and blood in vivo, as well as in cultured cells, under various pathophysiological situations such as muscular dystrophy, acute muscle regeneration, and hypoxia.
Figure: (A) Gating strategy used for isolation of intramuscular macrophages (CD45+ CD11b+ F4/80+ cells). (B) Percentages of these cells expressing the classical M1 (iNOS) and M2 (CD206) macrophage markers determined in standard dystrophic mice (mdx) versus dystrophic mice lacking Toll-like receptor 4 (mdx-TLR4-/-).
Gene Expression Analysis

Figure: Heatmap of gene expression data using an unsupervised hierarchical clustering analysis of differentially expressed genes in the diaphragms from control subjects (Control Dia) and patients who underwent prolonged mechanical ventilation (MV Dia) in the intensive care unit.
Confocal Microscopy

Figure: (A) Confocal imaging of oil red O-stained diaphragm from control subjects and patients who underwent prolonged mechanical ventilation (MV) in the intensive care unit, in order to evaluate intramyocellular fat content. (B) Three-dimensional reconstruction of confocal images used to quantify intramyocellular lipid droplet density and volume.
Quantitative Muscle Histology

Several methods of measurement such as point counting, color threshold segregation and integrated density analysis are coupled with unbiased systematic sampling to provide reliable quantitative data.
Figure: Point counting quantification of necrosis in dystrophic diaphragm. The grid is randomly overlaid on the tissue section image and the structures of interest (i.e., necrotic fibers) that are intersected by the grid are highlighted (green dots).
Muscle Physiology

In mice, electrical stimulation is applied to isolated muscle strips in an organ bath preparation or to entire muscles in situ at various frequencies, and force generation is directly determined. In humans, non-painful magnetic stimulation is applied to the phrenic nerves and respiratory pressures are used as a surrogate of respiratory muscle force output.
Figure: (A) In vitro organ bath preparation used to determine diaphragmatic force generating capacity. (B) Comparison of diaphragmatic force generation in wild-type (WT) and mdx mice. (C) Bilateral magnetic stimulation of the phrenic nerves to assess diaphragmatic force generation in humans.